Introduction to Glycosides
Glycosides are a fascinating category of compounds, which occupies a rather a significant place in the course of organic chemistry. They are vitally important for many biological functions and find lots of uses in medicine, food, and agriculture.
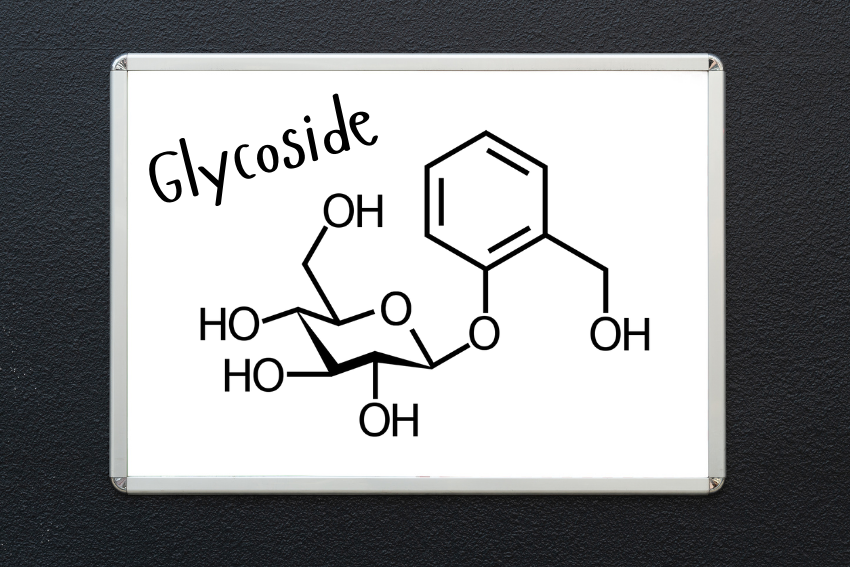
It consist of two primary components: a basic picture of a sugar molecule and a basic picture of a molecule which is not sugar. These two components are linked by glycosidic bond; the spatial arrangement of the link can be duplicated in either α or β configuration, depending on the position of the anomeric carbon.
The information presented in this article covers the general characteristics of glycosides in terms of structure and properties, as well as their utilization.
What are Glycosides?
Glycosides are organic compounds which contain glycosidic links, a special sort of linkage between a carbohydrate, known as the glycosides, and a non-carbohydrate substance, termed an aglycone. In its most characteristic form, the glycosidic linkage is made by elimination or a dehydrative coupling step in which the hydroxyl group of the sugar and a hydrogen atom of the aglycone are eliminated and an ether bond is formed. This bond connects the sugar and the aglycone and forms a single complex possessing of certain properties from the glycoside molecule.
These are widespread in nature, and numerous of them are obtained from plant raw materials. Lipids may play some major functions such as energy storage, serving as a signaling molecule and transmitting information between cells. Some glycosides are reported to possess appreciable pharmacological effects and a large number of these compounds are used in folk medicine for the treatment of various diseases.
Glycosides: Structure and Classification
These are organic compounds that consist of two parts: A sugar molecule and molecule of non-sugar, or aglycone’. The sugar moiety is frequently a monosaccharide or oligosaccharide whereas the non-sugar moiety is an alkaloid, steroid or carotenoid. Some of the descriptions for the use of the ingredients of glycosides are the glycosidic link, which is the connection between the sugar portion and the aglycone.
Depending on the structure and composition of glycosides there exist several classifications: Some of the most common categories I reckon includes:
- O-Glycosides: In these glycosides, the glycosidic bond is the example of the oxygen to carbon bond between oxygen of sugar and carbon of the aglycone. O-glycosides are widely distributed in natural products and are involved in many biologic processes occurring in organisms.
- N-Glycosides: Some of these glycosides have nitrogen atom in the glycosidic bond it forms with the sugar molecule. N-glycosides has various role in different biomolecules which are nucleosides and nucleotides.
- S-Glycosides: S-glycosides are in which the glycosidic bond is established between a sugar grouping and an aglycone by sulfur. Although these glycosides are minor, they play vital roles in the basic biological activities of the organism.
These glycosides can be divided based on sugar constituent; The major types of glycosides based on sugar constituent are as follows: There are monoglycoside, diglycoside, triglycoside, and polyglycosides are distinguished based on the number of sugar molecules found in the compound.
Properties of Glycosides
The properties of glycosides are unlike any organic compounds and there are still many aspects of them that are unknown to scientists. Some of their key properties include:
- Solubility: Glycosides are soluble in water than the aglycone complex In Glycoside, solubility in water is much higher than the aglycone complex. This solubility is attributable to the polar nature of the sugar component, at which the water molecule can form covalent bonds.
- Sweetness: Common examples of glycosides, the majority of which are sweet, contain saccharide moieties. This property is now of considerable importance in the food industry for its sweetening action.
- Chemical Stability: Glycosides in most of the cases are more chemically stable than their respective aglycones counterparts. The glycosides are superior to other compounds in terms of their resistance to hydrolysis and oxidative degradation since the sugar moiety shields the aglycone from degradation.
- Biological Activity: Various glycosides display diverse biological effects including antimicrobial, antiviral and anticancer effects. These activities are due to the steric behavior arising from the interactions between the sugar and aglycone components.
Applications of Glycosides
It uses in numerous areas including pharmacological, food industry and agriculture. Some of their key applications are:
- Medicine: Flavonoids have been reported to possess several biological activities, and they have been used as therapeutic agents because of their status as natural glycosides. In heart failure drug as digoxin or digitalin, and ouabain have been used also in the treatment of irregular he arterial rhythms. Other glycosides including the salicylic glycoside possess analgesic as well as anti-inflammatory properties useful in handling pain.
- Food and Beverages: Writing about their applications, the author notes that glycosides are often added to foods and drinks to provide sweetness and to enhance the taste. For example, the natural sweetener from stevia plant that replaces sugar, is sativoside. Also, sweeteners such as glycosides- vanillin and coumarin are used as a flavoring and fragrance agent in several products.
- Agriculture: It is established that glycosides participate in the plant’s defense system. For instance, the two types of surfactant that were discussed previously the natural surfactant, the saponin plays other roles of being an agent to guard plants from being fed on by herbivores as well as attack by pathogens are examples of this glycoside. The following are some of the functions include that they are also employed as pesticides synergists to enhance effectiveness of insecticides and fungicides for the intended pest control purpose.
Synthesis and Analysis of Glycosides
The mechanism of forming the glycoside assumes the formation of the glycosidic bond in the molecule between carbohydrate and non-carbohydrate components of the glycoside. There are several methods to synthesize glycosides, including:
- Glycosylation: The overall mechanism used in this method includes the participation of a sugar donor and an acceptor molecule. Enhancement of furans by glycosidase enzymes or transition metal catalysts normally execute the reaction.
- Nucleophilic Substitution: In this case, the group (sugar) departs to attach itself to create a glycosidic bond through the use of the hydroxyl group of the sugar moiety; the aglycone.
- Oxidative Glycosylation: This method involves oxidation of a primary alcohol on the sugar molecule and nucleophilic displacement with the aid of the aglycone.
There are a number of analytical methods that are relevant to identification and description of glycosides. Common techniques include:
- Mass Spectrometry: It defines the procedure used in the determination of molecular weight and other structural details of glycosides. Data presented in the form of an electrospray ionization mass spectrum provides information about the steric configuration of the glycosidic covalent bond and the identity of specific sugars.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: This method has also revealed the structure and conformation of glycosides in particular in organic compounds and other molecules. HMBC, a three-bond correlated spectroscopy, helps to define the relative stereochemistry of the glycosidic bond as well as the full structure of the molecule, whereas other two-dimensional COSY and NOESY are also important for achieving the similar results.
- Infrared (IR) Spectroscopy: It provides confirmation and details of the functional groups and the vibrational characteristics of glycosides. Certain characteristic peaks of the IR spectrum can be used for the determination of the sugar and aglycone fragments.
Why plant Glycosides are essential in plant metabolism?
In plants glycosides are involved in transport and storage of several chemical compounds, metabolism of xenobiotics and modulating the physiological processes. Protein glycosylation is confirmed as a major and critical post translational modification that improves the solubility, stability and activity of different proteins and enzymes in plants.
Another use of glycosides in plants is the storage of energy. For example, a plant providing an example can convert sugar from glucose and fructose into sukrin, known as glycoside, which it can use for instance within the tissues of the plant to be transported and stored in the plant fruits, roots and stems. This storage helps the plant to use the energy at night or at any time when the photosynthetic rate is low, or high energy usage is required.
They also take part in the detoxification and protection of plants. Most of the xenobiotics including pesticides and herbicides are known to have toxic impacts on plant life. Yet these compounds are metabolizable and detoxification by the plant through processes such as glycosylation (these compounds are conjugated with sugar molecules and can be easily excreted or stored without harmful effect to the plant cells.
Glycosides: Its Significance to Human Health
Flavonoids exist as glycosides in most of the plants and have been widely studied and reported because of their pharmacological actions. Some of which are important in medical uses such as cardiac glycosides which include digitoxin, digoxin used in managing heart failure and arrhythmia, and saponin, which has anti- inflammation, antioxidant, and anti-cancer properties.
Cardiac glycosides act in a way that they slow down the sodium potassium ATPase pump in cardiac muscles which enhances calcium uptake. This in return aids in the contractibility of heart muscles. This action assists in enhancing the function of the heart and in all round lowering on the burden on this organ thereby benefiting the disability.
Saponins can be described as a large group of compounds which are members of glycosides family; these compounds can be obtained from the root of ginseng and licorice, as well as from the Quillaja bark. They possess a design that can attach to cell membranes and subsequently disrupt the lipid bilayer and they are both endogenous and exogenous. This is why saponins have medicinal uses like; Cholesterol lowering agents, boosting the immune system, and cancer fighting things.
Glycosides in the Food Industry
In the food industry, massive amounts of glycosides are employed as sweetening agents, flavors, and food fortifiers. Sucrase is a non-reducing disaccharide glycoside which is the common enhance of non-reducing saccharides sweetening agents that prefer as the most preferred type of sweetening agent because of its heat stability. It is applied in many food products, soft drinks, baked products, confectioneries etc.
It also includes its uses as a sweetening agent, nutritive and flavoring agent in the context of the food industry. Vanillin glycoside for instance, derived from vanilla beans provides us with the natural vanilla flavor, in ice creams, cookies and cakes etc. Coumarin glycoside and benzaldehyde glycoside and the other analogous glycosides are also employed to provide flavors in foods and other food products in the same manner as described above.
Glycosides are used as sweetening agents, flavoring agents and are incorporated into functional foods since it serves as an emulsifying agent, stabilizing agent and a preservative in the preparation of food products. For example, pectin is polysaccharide glycoside, which is used to gel in the production of jam, jelly, fruit preserve and so on, and maltodextrin is partial hydrolyzed glycoside, is used to as a carrier of essential oil and flavor substance for food.
Glycosides in the Pharmaceutical Industry
The pharmaceutical industry also employs broad use of glycosides in formulation of the various drugs and therapeutic compounds. Because of their structural flexibility and possible pharmacological properties, glycosides are now considered a promising source of new leads in drug design.
It is understandable that one the most essential fields in the utilizing of glycosides in the context of the pharmaceutical industry is the synthesis of anticancer drugs. For example, the arabinoside glycosides; cytosine arabinoside and 1-β-D-arabinofuranosylcytosine are used in treating leukemia and other cancerous diseases. These glycosides act as anticancer because these leads to destruction of cells by preventing DNA and RNA synthesis.
The second crucial lookup of glycosides in pharmaceutics is the discovery of antiviral medicines that can act prophylactically. For example, acyclovir, a nucleoside glycoside, is used to provide treatment to populations infected with herpes simplex virus (HSV). The viral thymidine kinase phosphorylates it to acyclovir triphosphate, which is competing the natural deoxy nucleoside triphosphate, thus stopping the viral DNA synthesis.
Conclusion
Glycosides are one of the most important and widely varied groups of substances in terms of their structures, the effects which they produce, and the uses to which they are put.
Due to the difference of sugar and aglycone proportion, each interferon-alpha compound has different characteristics, for instance solubility, sweetness and chemical stability.
It is proposed to be on a large scale in medicinal, food and agriculture industries; thus are a subject of major importance in organic chemistry. This proposal has presented progress in synthesis with the help of sophisticated analysis to facilitate the study and application of glycosides.
Future research is bound to have more introduced glycoside derivatives that might help to solve some problems in different spheres of human activity – in the sphere of medicine, in food industry, in agriculture.